Lidocaine
Hydrochloride Injection, USP
Lidocaine hydrochloride is a short-acting amide-type local anesthetic with a rapid onset and short duration of action and contains no preservatives.
-
For infiltration and nerve block
Solution for Injection - Clear and colourless solution – Preservatives free – Single dose ampoule
Indication and usage
Lidocaine Hydrochloride Injection is indicated for production of local or regional anesthesia by infiltration techniques such as percutaneous injection, by peripheral nerve block techniques such as brachial plexus and intercostal and by central neural techniques such as lumbar and caudal epidural blocks, when the accepted procedures for these techniques as described in standard textbooks are observed.
Qualitative and quantitative composition
Lidocaine Hydrochloride Injection, USP solution is for single dose usage, and is Methyl Paraben Free (Preservative-Free).Lidocaine Hydrochloride Injection, USP solution is a sterile, nonpyrogenic, isotonic solution containing sodium chloride. The pH of this solution is adjusted to approximately 6.5 (5.0 to 7.0) with sodium hydroxide and/or hydrochloric acid
Nature and contents of container
Clear glass ampule, glass type 1, USP
Special precautions for storageStore at Room Temperature
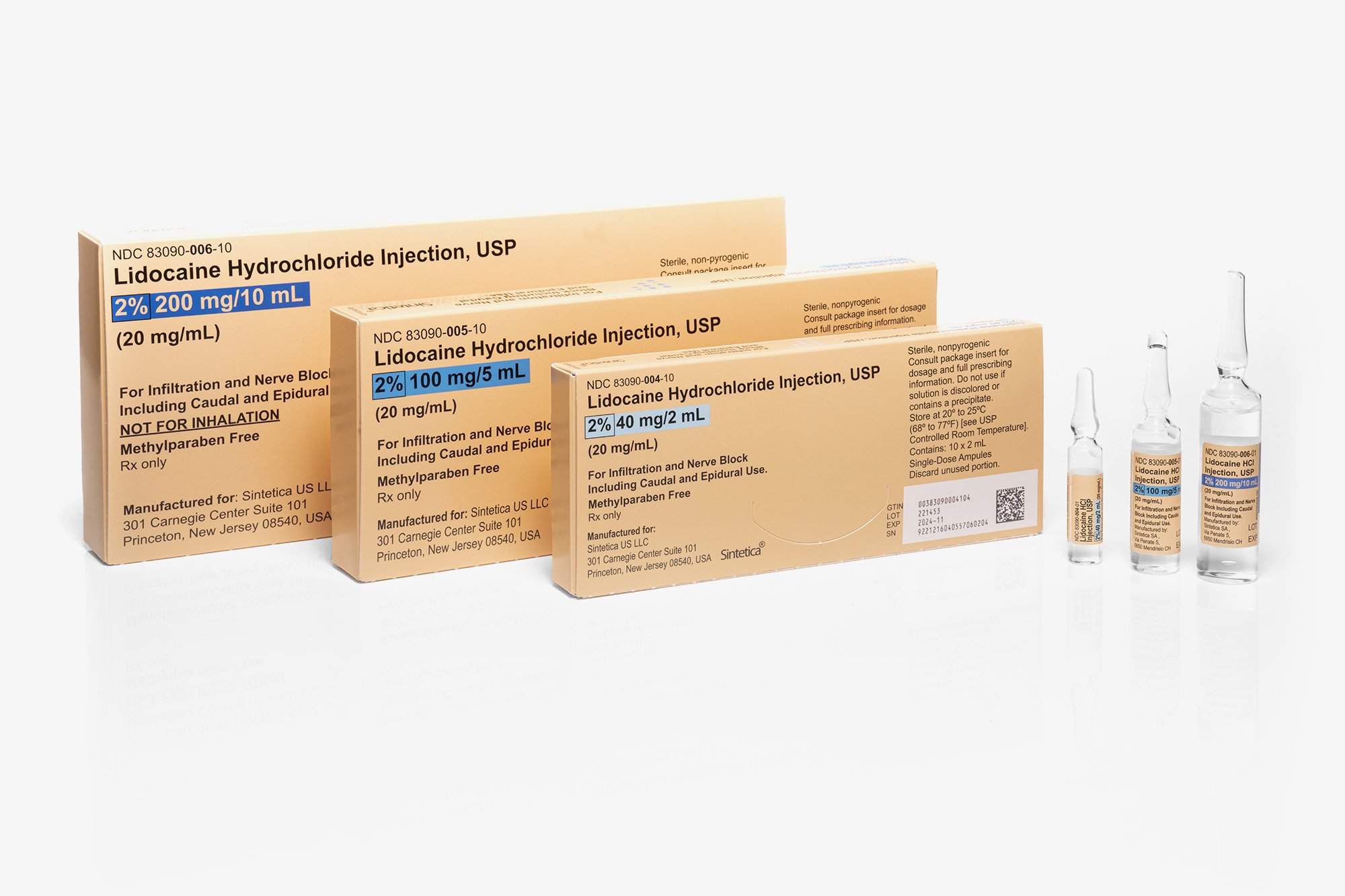
HOW SUPPLIED
Lidocaine Hydrochloride Injection, 1% (10mg per mL)
| Unit of sale | Strength | Each |
| NDC 83090-001-10 Unit of 10 |
1% (20 mg per 2 mL) (10mg per mL) |
2 mL Single-Dose Ampule |
| NDC 83090-002-10 Unit of 10 |
1% (50 mg per 5 mL) (10mg per mL) |
5 mL Single-Dose Ampule |
| NDC 83090-003-10 Unit of 10 |
1% (100 mg per 10 mL) (10mg per mL) |
10 mL Single-Dose Ampule |
Lidocaine Hydrochloride Injection, 2% (20mg per mL)
| Unit of sale | Strength | Each |
| NDC 83090-004-10 Unit of 10 |
2% (40 mg per 2 mL) (20mg per mL) |
2 mL Single-Dose Ampule |
| NDC 83090-005-10 Unit of 10 |
2% (100 mg per 5 mL) (20mg per mL) |
5 mL Single-Dose Ampule |
| NDC 83090-006-10 Unit of 10 |
2% (200 mg per 10 mL) (20mg per mL) |
10 mL Single-Dose Ampule |
To report suspected adverse reactions, contact Sintetica US, at +1-888-815-3345 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
Only for topical use and retrobulbar injection
Solution for Injection - Clear and colourless solution – Preservatives free – Single dose ampoule
Indication and usage
Lidocaine Hydrochloride Injection 4% is indicated for the production of topical anesthesia of the mucous membranes of the respiratory tract or the genito-urinary tract. It may be injected trans-tracheally to anesthetize the larynx and trachea, and it may be administered by retrobulbar injection to provide anesthesia for ophthalmic surgery.
Qualitative and quantitative composition
Each 1 ml contains 40.0 mg of lidocaine hydrochloride in water for injection
May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. pH 6.5 (5.0 to 7.0)
Nature and contents of container
Clear glass ampoule, glass type 1, USP
Special precautions for storage
Store at Room Temperature
HOW SUPPLIED
Lidocaine Hydrochloride Injection, 4% (40mg/mL)
| Unit of sale | Concentration |
| NDC 83090-007-10 pack of 10 single-dose ampules 5 mL ampule (200 mg/5 mL) |
4% 200 mg/5 mL (40mg per mL) |
To report suspected adverse reactions, contact Sintetica US, at +1-888-815-3345 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Product information
| NDC NUMBER | STRENGTH | UNIT SIZE | PACK QUANTITY | ABC | CARDINAL | MCK | BAR CODED | |
|---|---|---|---|---|---|---|---|---|
 | 83090-001-10 | 1% 20mg per 2mL | 2 mL | 10 ampules | 10288390 | 5905492 | 2928695 | ✔ |
 | 83090-002-10 | 1% 50mg per 5mL | 5 mL | 10 ampules | 10288351 | 5905500 | 2928778 | ✔ |
 | 83090-003-10 | 1% 100mg per 10mL | 10 mL | 10 ampules | 10288370 | 5905484 | 2928786 | ✔ |
 | 83090-004-10 | 2% 40mg per 2mL | 2 mL | 10 ampules | 10288300 | 5905526 | 2928802 | ✔ |
 | 83090-005-10 | 2% 100mg per 5mL | 5 mL | 10 ampules | 10288335 | 5905534 | 2928828 | ✔ |
 | 83090-006-10 | 2% 200mg per 10mL | 10 mL | 10 ampules | 10288249 | 5905518 | 2928851 | ✔ |
 | 83090-007-10 | 4% 200mg per 5mL | 5 mL | 10 ampules | 10288350 | 5905542 | 2928877 | ✔ |
Injectable
Preservative-free
Storage condition:
room temperature
Single use