Arsenic Trioxide Injection
For induction of remission and consolidation in patients with acute promyelocytic leukemia (APL) who are refractory to, or have relapsed from, retinoid anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
Flip-off seal
Sulfite-free
Latex-free
Preservative-free
Allergen-free
AP-rated
Flexibility
two strengths for suitable reconstitution
For intravenous use
Store at 25°C (77°F):
Excursion permitted from 15°C to 30°C (59°F-86°F)
Cytotoxic agent
Product information
| NDC NUMBER | STRENGTH | UNIT SIZE | PACK QUANTITY | ABC | CARDINAL | MCK | BAR CODED | |
|---|---|---|---|---|---|---|---|---|
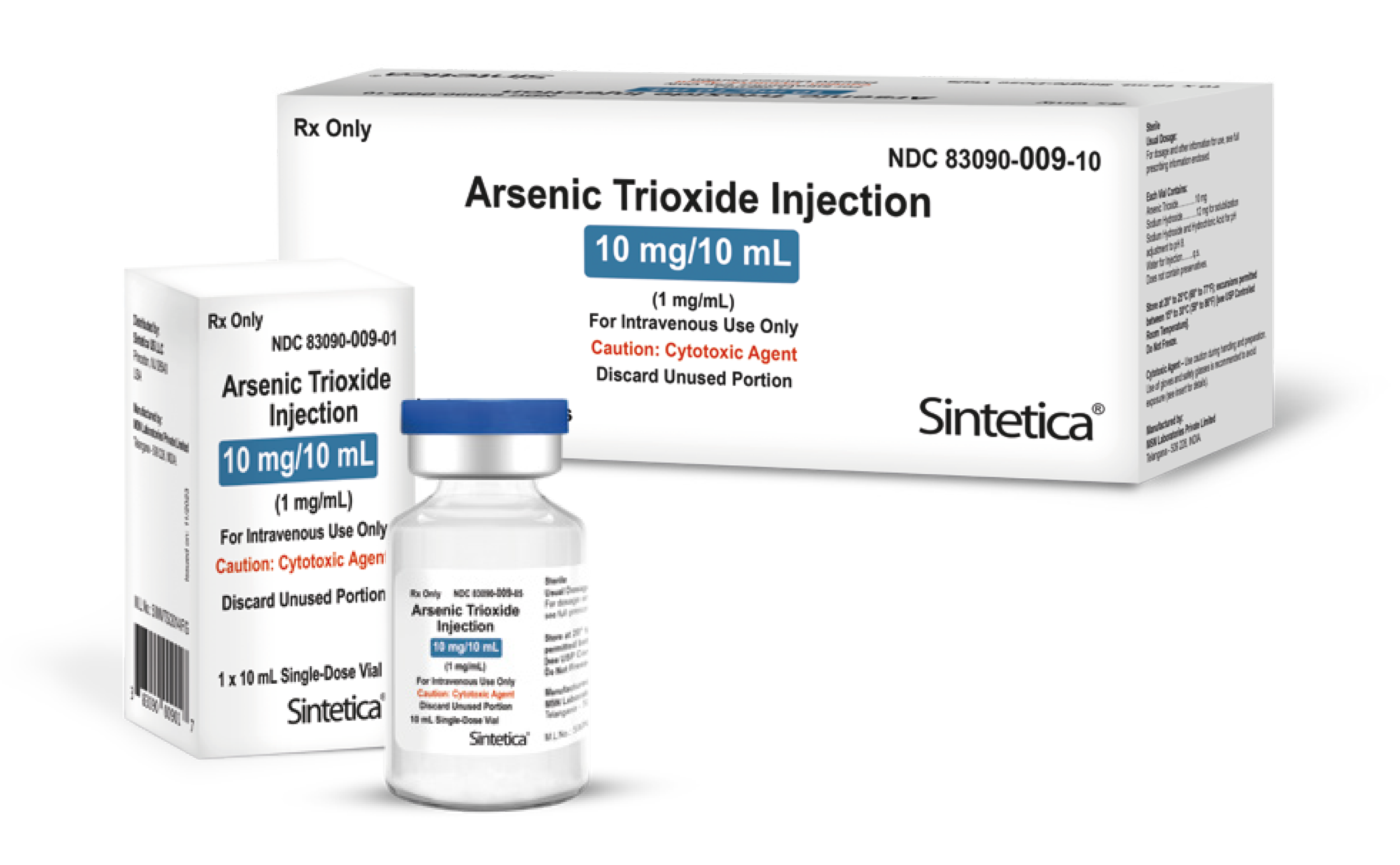
 | 83090-009-01 | 10 mg/10 mL (1 mg/mL) | 10 mL | 1 single-dose vial | 10288394 | 5906136 | 2929636 | ✔ |
 | 83090-009-10 | 10 mg/10 mL (1 mg/mL) | 10 mL | 10 single-dose vials | 10288189 | 5906144 | 2929768 | ✔ |
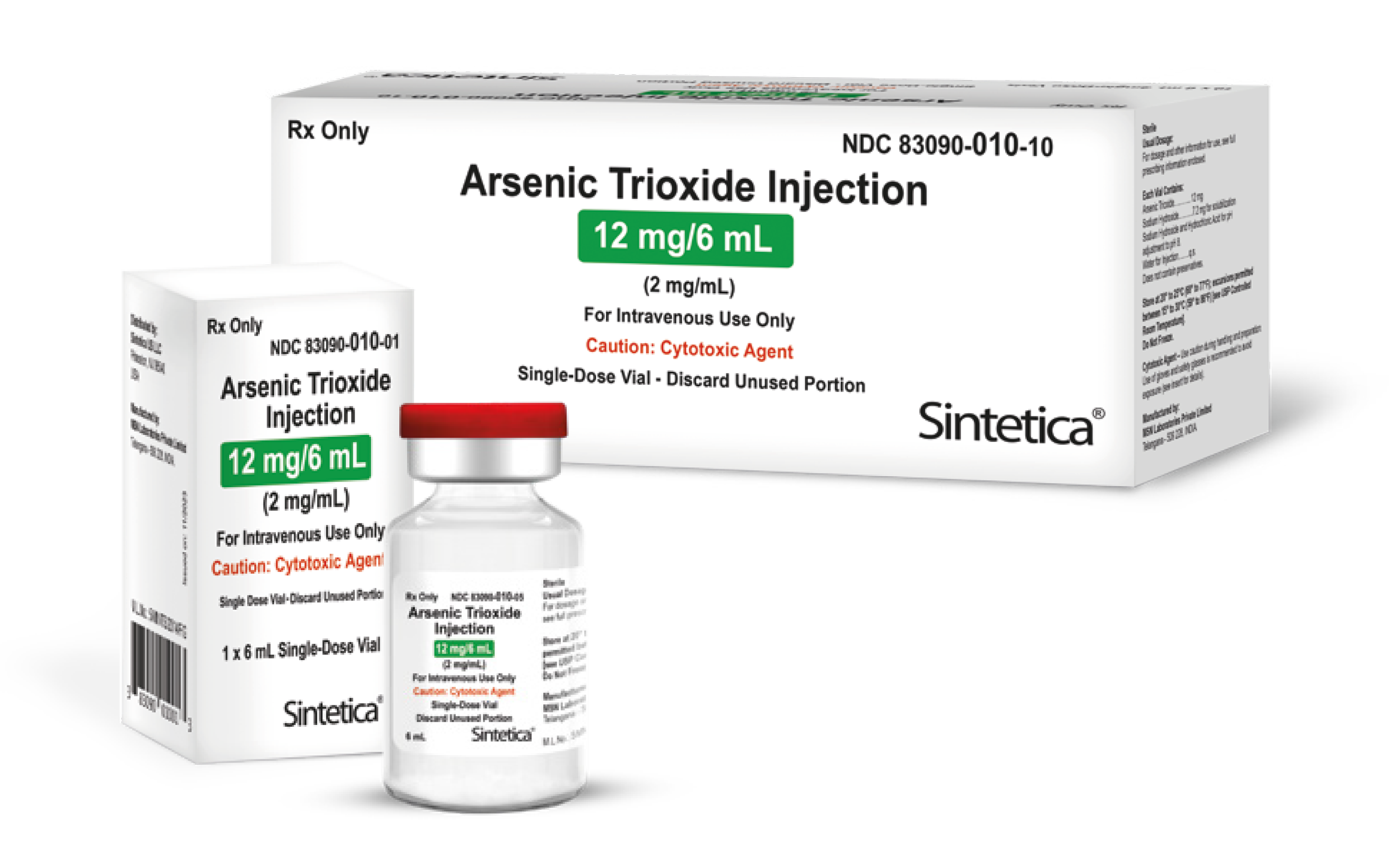
 | 83090-010-01 | 12 mg/6 mL (2 mg/mL) | 6 mL | 1 single-dose vial | 10288369 | 5906151 | 2929644 | ✔ |
 | 83090-010-10 | 12 mg/6 mL (2 mg/mL) | 6 mL | 10 single-dose vials | 10288340 | 5906169 | 2929776 | ✔ |

Important safety information
-
Arsenic trioxide injection is an arsenical indicated:
For induction of remission and consolidation in patients with APL who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or PML/RARalpha gene expression.
-
Relapsed or refractory APL:
Induction: Administer 0.15 mg/kg/day intravenously daily until bone marrow remission. Do not exceed 60 days.
Consolidation: Administer 0.15 mg/kg/day intravenously daily for 25 doses over a period up to 5 weeks.
-
Injection: 10 mg/10 mL (1 mg/mL) and 12 mg/6 mL (2 mg/mL) arsenic trioxide in single-dose vials.
-
Hypersensitivity to arsenic.
-
Hepatotoxicity: Monitor hepatic function tests at least twice weekly during induction and at least once weekly during consolidation. Withhold arsenic trioxide for certain elevations in AST, alkaline phosphatase and bilirubin and resume at reduced dose upon resolution.
Carcinogenesis: Arsenic trioxide is a human carcinogen. Monitor patients for the development of second primary malignancies.
Embryo-Fetal Toxicity: Can cause fetal harm. Advise of potential risk to a fetus and use of effective contraception.
-
The most common adverse reactions (> 30%) are nausea, cough, fatigue, pyrexia, headache, abdominal pain, vomiting, tachycardia, diarrhea, dyspnea, hypokalemia, leukocytosis, hyperglycemia, hypomagnesemia, insomnia, dermatitis, edema, QTc prolongation, rigors, sore throat, arthralgia, paresthesia, and pruritus.
-
Lactation: Advise not to breastfeed.
Renal Impairment: Monitor patients with severe renal impairment (creatinine clearance less than 30 mL/min) for toxicity when treated with arsenic trioxide; dose reduction may be warranted.
Hepatic Impairment: Monitor patients with severe hepatic impairment (Child-Pugh Class C) for toxicity when treated with arsenic trioxide.
To report SUSPECTED ADVERSE REACTIONS, contact Orbicular Pharmaceutical Technologies Private Limited at 1-888-370-4186 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
For more details please refer to the Full Prescribing Information